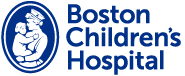Spotlight on Research
The first clinic for the treatment of genetic disorders was established in 1962 at “Children’s Hospital Medical Center” (as it was then known) in response to the advent of newborn screening for metabolic diseases. Our Division of Genetics was founded in 1965. While names have changed over the six decades since, our group has remained at the forefront of advancement in the field. We are today the multidisciplinary and collaborative Division of Genetics and Genomics at Boston Children’s Hospital. Discovery and innovation are embedded in our DNA. We are privileged to build on a distinguished history, and to benefit from a scale and breadth of expertise from lab bench to bedside, forming a virtuous circle with a unique potency to accelerate and enable innovation.
This section provides an overview of four domains in which our Division continues to spearhead discovery .
Advancing Genetics Diagnosis
The History of Genetic Screening
The identification of errors, or pathogenic mutations, in a patient’s genetic code is paramount to our quest to unravel congenital diseases, and fundamental to the realization of therapies or cures.
The 60-year career of Dr. Harvey Levy is a timeline that tracks the history of genetic screening in newborns and the expansion of the fields of molecular and metabolic genetics. Dr. Levy was an innovator at the bench and a leader in establishing specialized clinics and influencing public policy throughout the U.S
Dr. Levy’s research contributions include studies establishing the importance of newborn screening in preventing the intellectual disability of phenylketonuria (PKU), and descriptions of homocystinuria-related disorders that led to our present understanding of vitamin-related cofactors in the etiology of metabolic disorders.
The First Gene Map
Louis Kunkel has devoted four decades to a quest to diagnose and cure muscular dystrophy.
He is pictured here, standing with the six-foot-long, hand-drawn gene map, representing the area of the human genome where the dystrophin gene resides. Started in 1983, the drawing was published in 1986. It took science several technological revolutions to go from this first-ever depiction of a bit of genetic code to today’s high speed DNA sequencing – from Kunkel’s painstaking pencil scratches, to digital gene arrays.
You can read more about this story in “The Gene Hunter: Louis Kunkel’s 30-year quest to diagnose and cure muscular dystrophy” Harvard Magazine, March-April, 2011.

Finding Clues in the Clinic: Reverse Translation

Translational medicine refers to the process of bringing discoveries from the lab to use in patient treatment – hence, “from bench to bedside”. In 2016, Ming-Hui Chen, MD, Department of Cardiology and Division of Genetics and Genomics and Ryan Doan, PhD, then a post-doc in the Walsh Lab, now a principal investigator in the Division, published this high impact publication which demonstrates original discoveries about alternate splicing, poison exons, and variations in genetic expression across tissues.
Behind this seminal article is the story of a group of physicians, clinical researchers and basic scientists driven to decipher the atypical presentation of a large family with a brain and heart disorder — what mechanism was at work in these individuals? In the Walsh Lab, a post-doc research fellow was researching genetic mutations in the silent portion of the genome, in search for a specific human disorder engendered by this flaw. The willing participation of a patient family, the tenacity of the physician researcher, and the ingenuity of the bench scientists combined for a felicitous outcome. Such serendipity is uniquely enabled at a place like Boston Children’s Hospital, where Clinicians, Patients & Families, and Bench Researchers are integrated in collaborations of uncommon breadth and scope. Read Article >
Labs & Centers that are Advancing Genetic Diagnosis
Agrawal Lab >
Neonatology, genetic and molecular basis of congenital myopathies and orphan diseases; whole exome and genome sequencing.
Bodamer Lab >
Personalized medicine, Kabuki Syndrome, Lysosomal storage disorders (Niemann-Pick Type C) and spontaneous premature birth.
Doan Lab >
Genetics of neurodevelopment, autism, ADHD, OCD, dyslexia, bipolar disorder.
Flannick Lab >
the intersection of computer science, statistical genetics, and computational biology; current focus on diabetes.
Kunkel Lab >
Duchenne Muscular Dystrophy (DMD), Limb Girdle Muscular Dystrophy (LGMD) interstitial cystitis, autism spectrum disorder (ASD).
Manton Center for Orphan Diseases >
Research on new methods for understanding, diagnosing and treating rare genetic conditions.
Mochida Lab >
molecular basis of genetic disorders of human brain development; neuropathogenesis of congenital infections (esp. Zika); novel treatments for genetic and non-genetic developmental brain disorders.
O’Donnell-Luria Lab >
large-scale genomic and transcriptomic in rare disease diagnosis. Joint appointment with the Broad Institute.
Undiagnosed Disease Network >
A collaboration between Brigham and Women’s Hospital, Massachusetts General Hospital and Boston Children’s Hospital.
Walsh Lab >
Autism, human brain malformations, human brain evolution, single cell genomics.
Yu Lab >
Translational genomic medicine, autism.
Related Clinical Programs
Neurology, Genetics and Genomics,Metabolism Program, Brain Development and Genetics Clinic
Monica Wojcik, MD >
newborn medicine, genetics and genomics, novel disease gene discovery, Center for Mendelian Genomics at the Broad Institute of MIT and Harvard.
Relevant Publications
![]()
“The Gene Hunter: Louis Kunkel’s 30-year quest to diagnose and cure muscular dystrophy”
Harvard Magazine, March-April, 2011. Read Article >
Cell-Type-Specific Alternative Splicing Governs Cell Fate in the Developing Cerebral Cortex
DOI:https://doi.org/10.1016/j.cell.2016.07.025. Read Article >
Novel Therapies
If DNA is like software, can we just fix the code?
With Diagnostics, we study genes and genomes of patients to find the underpinnings of disorders. Then we search for ways to repair the break at the source with genetic therapies and other modalities in patient care.
Founded in 2008, the Manton Center for Orphan Disease Research at Boston Children’s Hospital is one of the first centers in the world solely devoted to the study of rare diseases. The Center leads and inspires a range of research projects, funding and awards, and collaborates with outside dedicated disease associations to facilitate the discovery and development of more effective diagnostic pathways and therapies for rare or unknown conditions.
In video at left, Dr. Tim Yu describes this process of how he and colleagues became the first group to diagnose a patient with a rare genetic syndrome, and to go on to develop a novel, customized medicine for that orphan disease. They were able to begin treating the patient within just one year.
You can read more about the journey of Dr. Yu, his team and the patient and her family in the article, “If DNA is like software, can we just fix the code?” in the MIT Tech Review.
Labs & Centers Working on Novel Therapies
Beggs Lab >
Genetics of congenital myopathies, neuromuscular disorders, translational gene therapy, and diagnosis of unsolved genetic disorders
Bodamer Lab >
Personalized medicine, Kabuki Syndrome, Lysosomal storage disorders (Niemann-Pick Type C) and spontaneaous premature birth.
Mochida Lab >
Molecular basis of genetic disorders of human brain development; neuropathogenesis of congenital infections (esp. Zika); novel treatments for genetic and non-genetic developmental brain disorders.
Yu Lab >
Translational genomic medicine, autism.
Clinical Programs Treating Patients with Novel Therapies
Angelman Syndrome
Contact Wen-Hann Tan, BMBS
Lysosomal Storage Disease Program (BoLD)
Walla Al Hertani, MD >
Lysosomal storage disorders and inborn errors of metabolism.
Gerard Berry, MD >
Mitochondrial Program, Metabolism Program, Precision medicine, carbohydrate metabolism, galactosemia, inositol metabolism in the brain, particularly during fetal development.
Deya Corzo, MD >
Division of Genetics and Genomics, Department of Pediatrics.
NF Research Initiative
David Miller, MD, PhD, Director, Neurofibromatosis Research Initiative.
PAL Clinic >
Stephanie Sacharow, MD, Program Director. Gene therapy for Phenylketonuria (PKU) and related conditions.
Relevant Publications
Lamin B2 Levels Regulate Polyploidization of Cardiomyocyte Nuclei and Myocardial Regeneration. Dev Cell. 2020 Apr 6;53(1):42-59.e11. doi: 10.1016/j.devcel.2020.01.030. Epub 2020 Feb 27. Read Article >
Emerging Technologies
“Progress in science depends on new techniques, new discoveries and new ideas, probably in that order”
– Sydney Brenner (1927–2019), geneticist, recipient of the 2002 Nobel Prize in Physiology and Medicine and dozens of other awards.
See related article. >
The history of science reveals again and again how technological innovations enable us to see what was previously invisible, enumerate that which had been incalculable or hear what had been silent. The horizons of genetic research have especially been expanded through the employment of new technologies, shedding light on Mendelian diseases as well as on more complex disorders. New technologies allow us to trick nature into showing us her secrets, permitting us to pinpoint unique mutations and seek therapies to correct them.
Bioinformatics
In this talk about detecting genetic variants in Type 2 diabetes, Dr. Jason Flannick delivers an overview of his work on the genetic architecture of Type 2 Diabetes and, more generally, how computational biology contributes to the understanding of disease biology.
Dr. Flannick and other faculty from our Division and the Broad Institute have been founding contributors to the Knowledge Portal Network. This project is a software platform and ecosystem of interconnected web portals that integrate, interpret, and present human genetic and genomic data to spark insights into complex diseases.
Single Cell Genomics
The brain is characterized by cellular diversity. Single-Cell Genomics is the study of the DNA or RNA that exists within a unique cell. Understanding how this diversity is programmed, damaged or mutated, is an essential piece in the puzzle of human disease.
In in July 2019, Dr. Walsh delivered a keynote address on his pioneering work in Single Cell Genomics for the Inaugural Mind Brain Behavior Symposium at the Columbia University Zuckerman Institute.
Labs & Centers Working with Emerging Technologies
Choudhury Lab >
Molecular cardiology.
Flannick Lab >
The intersection of computer science, statistical genetics, and computational biology; current focus on diabetes. Joint appointment with the Broad Institute.
Lee Lab >
Computational genomics and bioinformatic approach to characterization of genomic variation and functional changes associated with human disease.
O’Donnell-Luria Lab >
Large-scale genomic and transcriptomic in rare disease diagnosis. Joint appointment with the Broad Institute.
Structural and functional disorders of the human brain, single cell genomics
Relevant Publications
Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat Neurosci. 2015 May;18(5):637-46. doi: 10.1038/nn.3980. Read Article >
The BabySeq project: implementing genomic sequencing in newborns
BMC Pediatrics volume 18, Article number: 225 (2018). Read Article >
Improving the Understanding of Genetic Variants in Rare Disease With Large-scale Reference Populations. JAMA Insights Genomics and Precision Health. August 30, 2019. Read Article >
Developmental Genetics
We start from the perspective that the critical path to a solution begins from a comprehensive understanding of the problem at its roots and its context. In Developmental Genetics, we focus on the fundamental processes that occur as a single cell manifests into an organ or organism. When these processes go awry, we are looking at the anatomy of genetic disease and the downstream effects on the entire organisms.
We deploy discovery-driven basic research in multiple model organisms (eg, C. elegans, zebrafish, mice, macaques and human cells in culture). By constructing the developmental biology of tissues such as skeletal muscle and brain, dissecting the function of individual genes in these organ tissues, we arrive at an intellectual framework in which to interpret disease pathology and eventually treat genetic disorders. In our labs, we investigate mutations in a particular gene and observe how they affect various model organs in a range of model systems.
Brain Abnormalities
Some of our research focuses on the mutations associated with abnormalities in the brain. We trace how neurons attain their targets and migrate accurately to their apposite anatomical destinations. Disruptions or errors in these processes may lead to conditions such as: autism spectrum disorders, brain malformation disorders, lissencephaly, PKU, etc. (See: Engle, Heiman, Walsh).
Muscle & Skeletal Diseases
Errors that occur in genes responsible for the proper development of skeletal muscle fibers or muscle stem cells lead to myopathies and dystrophies that have been the focus of our research for generations. (See: Beggs, Gussoni, Heiman, Kunkel.)

Brain organoids are “mini-brains” derived from human pluripotent stem cells. They sufficiently resemble the embryonic human brain to serve as a model to study human development and disorders.
Images courtesy of Xuyu Qian, PhD, Research Fellow, the Walsh Lab
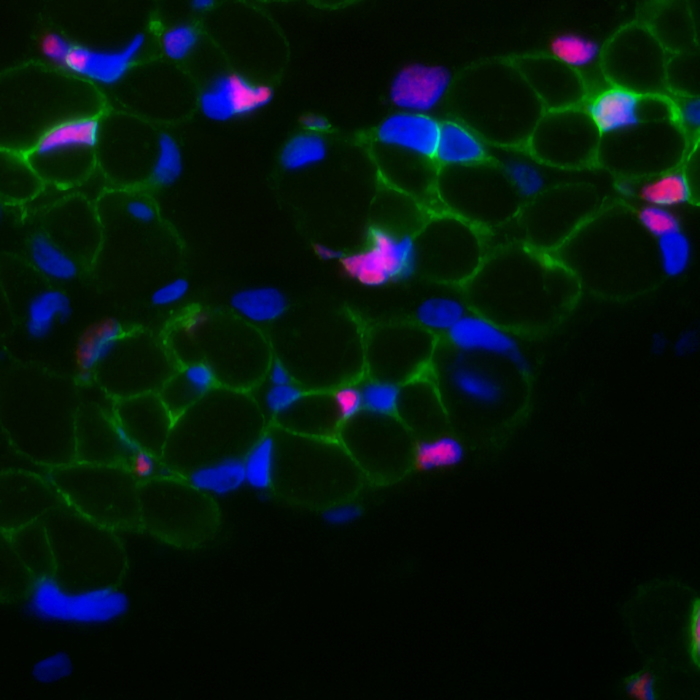
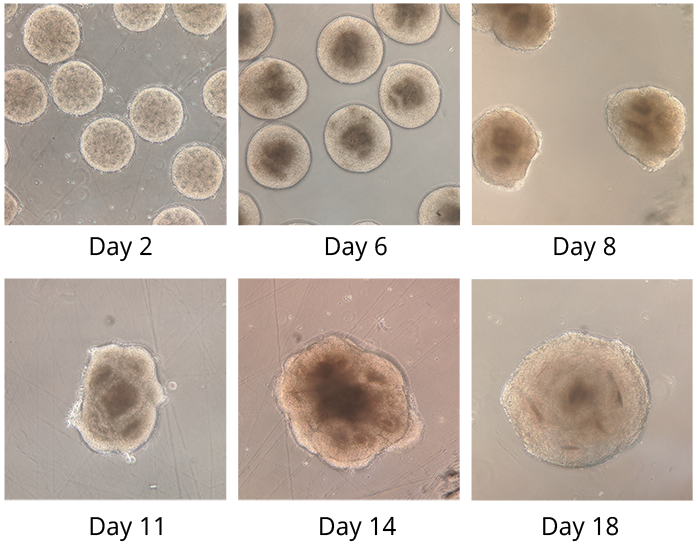
Image above: developing muscle with stem cells in red, located nearby nascent myofibers in green. By Anete Rozkalne, Gussoni Lab.

Image above: immunostaining of human adult muscle, demonstrating mature myofibers. By Emanuela Gussoni, PhD, Gussoni Lab.
Labs & Centers
Beggs Lab >
The molecular biology of skeletal muscle and its disruption in diseases of muscle weakness. Model systems: zebrafish, mouse, cell culture.
Engle Lab >
Congenital cranial dysinnervation disorders and disorders of axon guidance. Model systems: mouse, zebra fish.
Gussoni Lab >
Muscle stem cells seeking strategies to slow the progression of and improve muscle function for muscular dystrophy. Model systems: mouse, human stem cells.
Heiman Lab >
Cell-cell interactions during development, especially between neurons and glia. Model system: C. elegans.
Kunkel Lab >
Duchenne Muscular Dystrophy (DMD), Limb Girdle Muscular Dystrophy (LGMD) interstitial cystitis, autism spectrum disorder (ASD). Model systems: mouse, zebrafish, cell culture.
Walsh Lab >
Development and organization of the mammalian cortex. Model systems: mouse, ferret.
Relevant Publications
What Happens When It Goes Wrong? Using Human Genetics to Understand Human Brain Development and Evolution
![]() Read Article >
Read Article >
Evolutionary Changes in Transcriptional Regulation: Insights into Human Behavior and Neurological Conditions. Annual Review of Neuroscience.
![]() Read Article >
Read Article >
Somatic mutation in single human neurons tracks developmental and transcriptional history.
Science [cover illustration], 350(6256), 94-8. PMID: 2643012. DOI: 10.1126/science.aab1785
Aging and neurodegeneration are associated with increased mutations in single human neurons.
Science, 359(6375), 555-559
Dendrites with specialized glial attachments develop by retrograde extension using SAX-7 and GRDN-1. Development. 2020 Feb 17;147(4):dev180448. doi: 10.1242/dev.180448.
Morphogenesis of neurons and glia within an epithelium. Development. 2019 Feb 20;146(4):dev171124. doi: 10.1242/dev.171124.
Posterior Neocortex-Specific Regulation of Neuronal Migration by CEP85L Identifies Maternal Centriole-Dependent Activation of CDK5. Neuron. 2020 Apr 22;106(2):246-255.e6. doi: 10.1016/j.neuron.2020.01.030. Epub 2020 Feb 24.
The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles. Cell Rep. 2018 Jul 24;24(4):973-986.e8. doi: 10.1016/j.celrep.2018.06.100.
Somatic Mutations Activating the mTOR Pathway in Dorsal Telencephalic Progenitors Cause a Continuum of Cortical Dysplasias. Cell Rep. 2017 Dec 26;21(13):3754-3766. doi: 10.1016/j.celrep.2017.11.106.
CD82 Is a Marker for Prospective Isolation of Human Muscle Satellite Cells and Is Linked to Muscular Dystrophies. Cell Stem Cell. 2016 Dec 1;19(6):800-807. doi: 10.1016/j.stem.2016.08.006. Epub 2016 Sep 15.
Repression of phosphatidylinositol transfer protein α ameliorates the pathology of Duchenne muscular dystrophy. Proc Natl Acad Sci. 2017 Jun 6;114(23):6080-6085. doi: 10.1073/pnas.1703556114. Epub 2017 May 22.